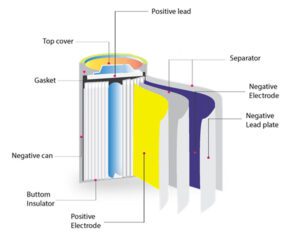
Basic Parts of battery
Batteries are electrochemical devices that convert chemical energy into electrical energy. While there are different types of batteries with varying designs, they typically consist of several basic components:
- Electrodes: Batteries have two electrodes: a positive electrode (cathode) and a negative electrode (anode). The cathode is typically the electrode where reduction (gain of electrons) occurs during the battery’s operation, while the anode is where oxidation (loss of electrons) takes place.
- Electrolyte: The electrolyte is a medium that allows the movement of ions between the cathode and anode while preventing the direct flow of electrons. It can be a liquid, gel, or solid, depending on the type of battery. The electrolyte facilitates the transfer of ions and maintains charge balance during the battery’s operation.
- Separator: The separator is a permeable material positioned between the cathode and anode. Its primary function is to prevent direct contact between the electrodes while allowing the passage of ions. The separator helps to prevent short circuits within the battery and maintain the proper flow of charge.
- Collector: The collector is a conductive material that connects the electrode to the external circuit. It allows the flow of electrons between the electrode and the external circuit, enabling the battery to deliver electrical energy to devices or systems.
- Terminal: The terminals are the points of connection on the battery where external electrical devices or systems can be connected. The positive terminal (also called the cathode terminal) and the negative terminal (also called the anode terminal) allow the transfer of electrical energy between the battery and external components.
- Casing: The casing or housing encloses the internal components of the battery, providing physical protection and insulation. It is typically made of durable materials such as plastic or metal, ensuring the safety and integrity of the battery.
- These basic components work together to enable the electrochemical reactions that produce and store electrical energy within the battery.
- The specific materials and designs of these components vary depending on the type of battery, such as lithium-ion, lead-acid, or nickel-metal hydride, each with its own unique chemistry and construction.
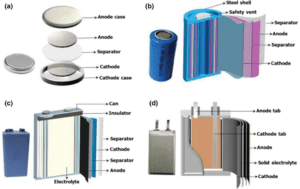
Types of Batteries
There are several types of batteries available today, each with its own unique chemistry and characteristics. Here are some common types:

Alkaline Batteries:
- Alkaline batteries are widely used in various devices such as remote controls, toys, and flashlights.
- They use an alkaline electrolyte (usually potassium hydroxide) and a zinc anode, and a manganese dioxide cathode. Alkaline batteries are known for their long shelf life, relatively high energy density, and cost-effectiveness.
Lithium-Ion Batteries:
- Lithium-ion (Li-ion) batteries are prevalent in portable electronics, electric vehicles, and renewable energy storage systems. They employ a lithium compound as the cathode (e.g., lithium cobalt oxide, lithium iron phosphate) and a carbon-based material as the anode (typically graphite). Li-ion batteries are known for their high energy density, lightweight design, and rechargeable nature.

Lead-Acid Batteries:
- Lead-acid batteries have been widely used in automotive applications and as backup power systems.
- They use a liquid sulfuric acid electrolyte, a lead dioxide cathode, and a lead anode. Lead-acid batteries are known for their robustness, low cost, and ability to deliver high currents. However, they have relatively lower energy density compared to some other types.
Nickel-Cadmium Batteries:
- Nickel-cadmium (Ni-Cd) batteries have been commonly used in portable electronics, though they are less prevalent today due to environmental concerns related to cadmium. They employ a nickel oxide hydroxide cathode, a cadmium anode, and an alkaline electrolyte. Ni-Cd batteries are known for their high discharge currents, good performance at low temperatures, and long cycle life.
Nickel-Metal Hydride Batteries:
- Nickel-metal hydride (NiMH) batteries are often used as an alternative to Ni-Cd batteries. They use a hydrogen-absorbing alloy as the anode (instead of cadmium) and a nickel oxide hydroxide cathode. NiMH batteries offer higher energy density than Ni-Cd batteries, are more environmentally friendly, and have less susceptibility to memory effects.
Lithium Polymer Batteries:
- Lithium polymer (LiPo) batteries are a variation of lithium-ion batteries but use a solid or gel-like electrolyte instead of a liquid.
- This allows for flexible and lightweight designs, making LiPo batteries popular in applications such as smartphones, tablets, and wearable devices. They offer high energy density and are rechargeable.
Solid-State Batteries:
- Solid-state batteries are an emerging technology that uses solid-state electrolytes instead of liquid or gel electrolytes.
- They offer improved safety, higher energy density, and faster charging compared to traditional lithium-ion batteries. Solid-state batteries are being developed for various applications, including electric vehicles and portable electronics.

- These are just a few examples of battery types, and ongoing research and development continue to explore new chemistries and designs to improve energy storage capabilities, safety, and sustainability.

Battery Design
- The battery design is at the forefront of energy storage innovation, enabling advancements across multiple industries and paving the way for a sustainable future.
- From the continuous improvements in lithium-ion batteries to the emergence of solid-state batteries and the exploration of alternative chemistries, researchers are pushing the boundaries of what batteries can achieve.
- As these innovations progress, we can expect longer-lasting, safer, and more efficient energy storage solutions that will revolutionize our daily lives and accelerate the transition toward a clean energy economy.
Chemistry of Batteries
Batteries are electrochemical devices that convert chemical energy into electrical energy through redox (reduction-oxidation) reactions. They consist of two electrodes, an electrolyte, and a separator.
- Electrodes:
- Anode: The anode is the electrode where oxidation occurs, releasing electrons. In most batteries, the anode is composed of a metal or a material capable of hosting ions, such as lithium or zinc.
- Cathode: The cathode is the electrode where reduction takes place, accepting electrons. It is usually made of a different material than the anode and can vary depending on the type of battery. Common cathode materials include transition metal oxides, such as cobalt oxide or nickel oxide.
- Electrolyte:
- The electrolyte is a medium that allows the movement of ions between the anode and cathode. It can be in liquid, gel, or solid form, depending on the battery type. The electrolyte facilitates the flow of ions while preventing the direct contact of the anode and cathode to avoid short circuits.
- Separator:
- The separator is a porous material placed between the anode and cathode to prevent electrical contact while allowing the passage of ions. It is typically made of a polymer or ceramic material.
Battery Types
- Primary Batteries:
- Primary batteries are non-rechargeable batteries that use irreversible chemical reactions. Once the reactants are consumed, the battery cannot be used again. Examples include alkaline batteries and zinc-carbon batteries.
- Secondary Batteries:
- Secondary batteries are rechargeable batteries that undergo reversible chemical reactions, allowing them to be recharged multiple times. The reaction can be reversed by applying an external electrical current. Common examples include lithium-ion batteries, lead-acid batteries, and nickel-metal hydride (NiMH) batteries.
Chemistry of Common Battery Types:
- Lithium-ion Batteries:
- Lithium-ion batteries utilize lithium ions that move between the anode and cathode during charge and discharge cycles. The anode is typically made of graphite, which intercalates lithium ions, while the cathode consists of metal oxides, such as lithium cobalt oxide or lithium iron phosphate.
- Lead-Acid Batteries:
- Lead-acid batteries are widely used in automotive applications. They employ lead dioxide as the positive electrode (cathode), lead as the negative electrode (anode), and a sulfuric acid electrolyte.
- Nickel-Metal Hydride (NiMH) Batteries:
- NiMH batteries consist of a nickel-based positive electrode (cathode), a hydrogen-absorbing alloy as the negative electrode (anode), and a potassium hydroxide electrolyte.
- These are just a few examples, and there are numerous other battery chemistries, each with its own unique characteristics and applications.
- Ongoing research continues to explore new materials and designs to enhance battery performance, energy density, and safety.
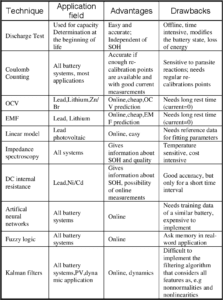
Applications of Battery
There are various types of batteries available today, each with its own unique chemistry and characteristics. Here are some common types of batteries and their applications:
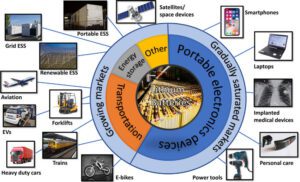
- Alkaline Batteries:
- : Remote controls, toys, flashlights, portable radios, clocks, and various low-drain devices.
- Lithium-Ion Batteries:
- Portable electronics (smartphones, laptops, tablets), electric vehicles, hybrid vehicles,
- Renewable energy storage systems, power tools, and medical devices.
- Lead-Acid Batteries:
- Automotive starting batteries, backup power systems, uninterruptible power supply (UPS), electric wheelchairs, golf carts, and marine applications.
- Nickel-Cadmium Batteries:
- Cordless power tools, emergency lighting, portable electronic devices (cameras, portable radios), and backup power systems.
- Nickel-Metal Hydride Batteries
- Cordless phones, digital cameras, portable electronic devices, hybrid vehicles, and medical devices.
- Lithium Polymer Batteries:
- Smartphones, tablets, wearable devices, remote-controlled vehicles, drones, portable medical devices, and electronic cigarettes.
- Solid-State Batteries:
- Electric vehicles, portable electronics, wearable devices, aerospace applications, and grid-scale energy storage.
- Zinc-Carbon Batteries:
- Low-power devices (remote controls, flashlights, wall clocks), toys, and smoke detectors.
- Silver-Oxide Batteries: Applications: Watches, calculators, hearing aids, medical devices, and small electronics.
- Rechargeable vs. Non-Rechargeable:
- Batteries can be categorized into rechargeable and non-rechargeable types.
- Rechargeable batteries, such as lithium-ion, nickel-cadmium, and nickel-metal hydride, are designed for multiple charging and discharging cycles, making them suitable for long-term use.
- Non-rechargeable batteries, such as alkaline and zinc-carbon, are meant for single use and are discarded after discharge.
Summary
- It’s important to note that battery technology is continually evolving, with ongoing research and development focused on improving energy density, cycle life, safety, and environmental sustainability. New types of batteries and advancements in existing technologies are being explored to meet the increasing demand for energy storage in various industries and applications.
- To increase the life of battery cooling techniques are used in many industrial applications and electrical vehicles. CFD simulations have been carried out for battery thermal management systems (BTMS) for different ambient conditions

