Quality of Water
- Water is an essential resource for all living beings, and it’s used in various daily activities such as cooking, cleaning, and drinking. However, the quality of water can vary, and one important factor to consider is its hardness
- Hard water refers to water that contains high levels of minerals such as calcium and magnesium. This can lead to various problems, including damage to appliances, skin irritation, and decreased efficiency of cleaning products. In this blog post, we will discuss the hardness of water, its causes, and its effects.

What is water hardness?
- Water hardness is the number of dissolved minerals, primarily calcium and magnesium ions, in water. Hardness is typically measured in parts per million (ppm) or milligrams per liter (mg/L). The higher the concentration of these minerals, the harder the water is considered to be
- What causes water hardness? Water hardness is primarily caused by the natural presence of minerals in the earth’s crust. When water passes through rocks and soil, it can dissolve minerals such as calcium and magnesium.
- Other factors that can contribute to water hardness include agricultural and industrial runoff, as well as the use of certain types of water treatment processes.
How to measure the hardness of Water
- Water hardness refers to the number of dissolved minerals, primarily calcium and magnesium, in water. While these minerals are not harmful to human health, they can affect the taste and appearance of drinking water

- There are a few different ways to measure the hardness of water, but one of the most common methods is to test the concentration of dissolved minerals, primarily calcium and magnesium, in the water
- Here are the basic steps for measuring water hardness using a simple test kit:
-
- Obtain a water hardness test kit: These are readily available online or at most hardware or home improvement stores.
- Collect a water sample: Fill a clean container with a sample of the water you want to test. Be sure to follow the instructions for the specific test kit you are using, as some may require a larger or smaller sample size.
- Add the test reagents: Follow the instructions on the test kit to add the appropriate amount of reagent to the water sample. This may involve adding drops of a chemical solution or using test strips that change color in response to the mineral content of the water.
- Read the results: After adding the reagents, wait for the designated amount of time before reading the results. The test kit should provide a chart or scale that allows you to compare the color or other visual indicator of your sample to the appropriate level of water hardness.
- Note that this method provides a rough estimate of water hardness, and may not be as accurate as more sophisticated laboratory testing. If you have concerns about the quality of your drinking water, you may want to consider having it tested by a professional water testing service.
Water Hardness Scale
- In general, the hardness of drinking water is measured in terms of its concentration of calcium carbonate (CaCO3) in milligrams per liter (mg/L) or parts per million (ppm). The following guidelines are commonly used to classify water hardness:
-
- Soft water: less than 60 mg/L (or ppm) CaCO3
- Moderately hard water: 60-120 mg/L (or ppm) CaCO3
- Hard water: 120-180 mg/L (or ppm) CaCO3
- Very hard water: more than 180 mg/L (or ppm) CaCO3

- In terms of drinking water, any level of hardness within these ranges is generally considered safe for human consumption. However, if the water is excessively hard, it may require additional treatment or filtration to improve its taste and reduce mineral buildup in pipes and appliances
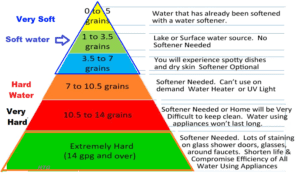
Difference Between Soft and Hard water
- The main difference between soft and hard water is the amount of dissolved minerals, primarily calcium and magnesium, that they contain.
- Soft water has low levels of these minerals, while hard water has high levels. This difference can have a number of effects on the properties and uses of the water.
- Here are some of the key differences between soft and hard water:
- Soap lathering: Soft water lathers easily with soap, while hard water requires more soap to produce the same amount of lather. This is because the dissolved minerals in hard water react with soap to form a sticky, scum-like substance that is difficult to rinse away.
- Mineral buildup: Hard water can lead to the buildup of mineral deposits, or scale, on pipes, fixtures, and appliances. This can reduce water flow and efficiency, and may require more frequent cleaning and maintenance.
- Taste: Soft water is often described as having a clean, fresh taste, while hard water can have a slightly bitter or metallic taste.
- Laundry: Soft water is generally better for washing clothes, as it allows detergents to dissolve more completely and rinse away more easily. Hard water can leave clothes looking dingy and feeling stiff.
- Health: While hard water is not harmful to human health, some studies suggest that it may contribute to certain health problems, such as eczema, and may make it harder for some people to absorb certain nutrients from food. Soft water, on the other hand, has not been associated with any negative health effects.
Effects of hard water
The presence of calcium and magnesium ions in hard water can cause various problems, including:
- Damage to appliances: Hard water can cause mineral buildup in appliances such as dishwashers and washing machines, which can reduce their efficiency and lifespan.
- Skin irritation: Hard water can dry out the skin and cause irritation, leading to conditions such as eczema.
- Decreased efficiency of cleaning products: Hard water can reduce the effectiveness of cleaning products, as the minerals can react with the active ingredients, preventing them from working effectively.
- Stains and buildup: Hard water can cause stains on clothes and dishes, as well as buildup on faucets and other surfaces.

How to reduce the water hardness?
- There are various methods for testing water hardness, including using test strips, laboratory analysis, and electronic testing devices. Test strips are a convenient and affordable option, while laboratory analysis provides the most accurate results.
- There are various methods for treating hard water, including:
-
- Water softeners: Water softeners work by replacing the calcium and magnesium ions with sodium or potassium ions, effectively reducing the hardness of the water.
- Reverse osmosis: Reverse osmosis is a water treatment process that involves passing water through a semi-permeable membrane to remove minerals and other impurities.
- Distillation: Distillation involves boiling water and collecting the steam, which is then condensed to remove impurities, including minerals.
- The hardness of water is an important factor to consider when it comes to the quality of water. Hard water can cause various problems, including damage to appliances, skin irritation, and decreased efficiency of cleaning products. There are various methods for testing and treating hard water, including water softeners, reverse osmosis, and distillation. By understanding the hardness of your water and taking appropriate measures to treat it, you can ensure that you have access to clean and safe water for all your daily needs.
What is the TDS (Total Dissolved Solids) of water
TDS (Total Dissolved Solids) is a measure of the total amount of dissolved substances in water, including minerals, salts, and other inorganic and organic compounds. There are several ways to measure TDS in water, including:
TDS meter:
- A TDS meter is a device that measures the electrical conductivity of water and calculates the TDS based on the conductivity.
- TDS meters are relatively inexpensive and easy to use, and they provide a quick and accurate measurement of TDS.
Evaporation method:
- This method involves evaporating a measured amount of water and measuring the residue left behind.
- The residue is then dried and weighed, and the TDS is calculated based on the weight of the residue.
- This method is more time-consuming and less accurate than using a TDS meter.
Gravimetric method
- This method involves filtering a measured amount of water through a filter, drying the filter, and weighing it
- The weight of the filter is then subtracted from the weight of the filter and residue, and the TDS is calculated based on the weight of the residue. This method is more accurate than the evaporation method but requires more time and equipment.
Titration method:
- This method involves adding a reagent to a measured amount of water and titrating the solution until the endpoint is reached. The TDS is then calculated based on the volume and concentration of the reagent used. This method is more complicated and requires more equipment and expertise than the other methods.
Summary
- Overall, using a TDS meter is the easiest and most convenient method for measuring TDS in water
- However, other methods may be necessary for more accurate or precise measurements, depending on the specific requirements of your application.


